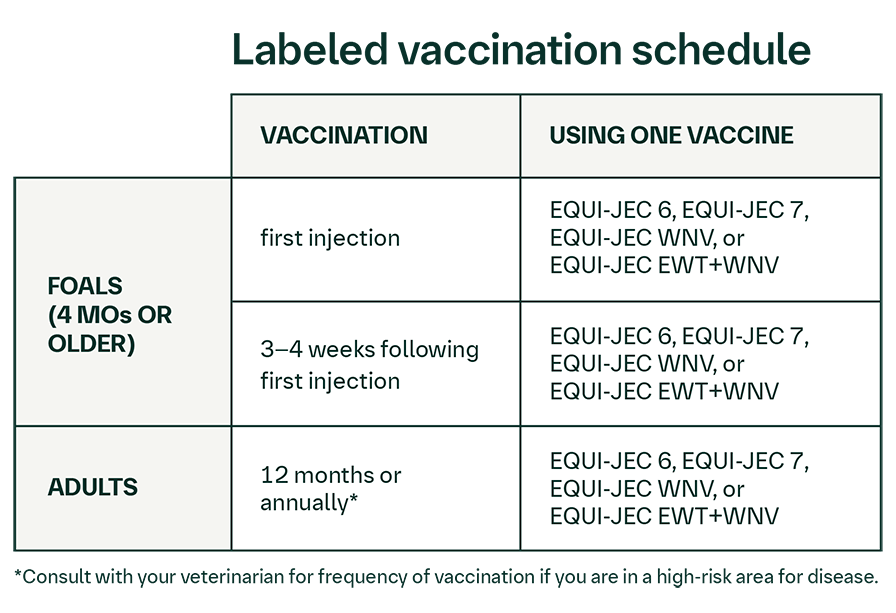
Shake well before use. Using aseptic technique, vaccinate horses intramuscularly with a 1-mL dose. Administer a second 1-mL dose intramuscularly in 3 to 4 weeks using a different injection site. The need for annual booster vaccinations has not been established for this product; consultation with a veterinarian is recommended.
Precautions: Store at 35-46ºF (1-8ºC). Do not freeze. Do not mix with other products. Do not vaccinate within 21 days before slaughter. In case of anaphylactoid reaction, administer epinephrine. In case of human exposure, contact a physician.
See product insert for administration information.
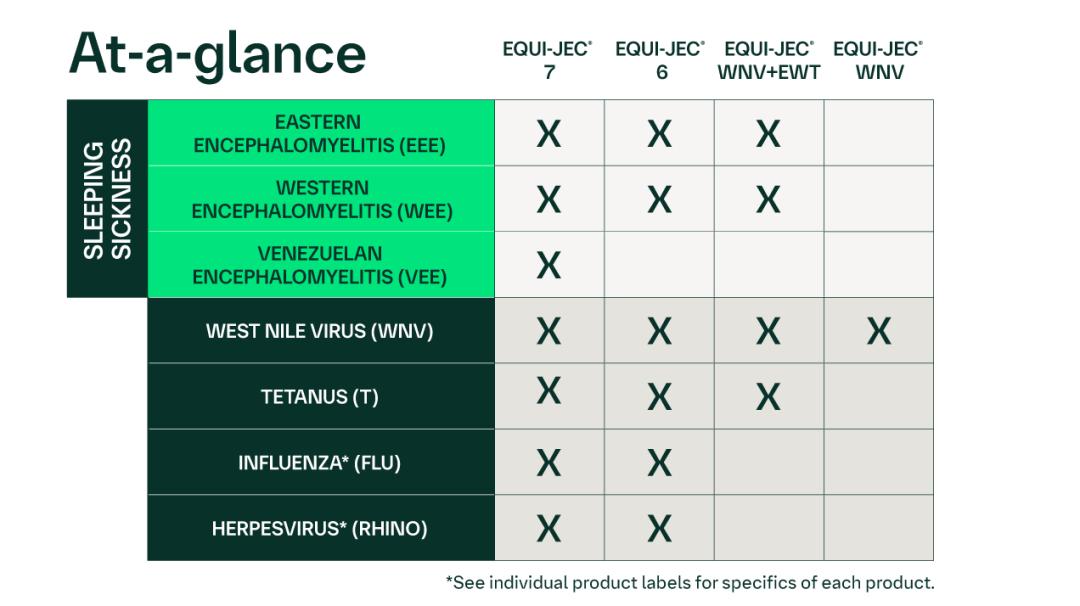
EQUI-JEC 7 has been shown to be effective for the vaccination of healthy horses 4 months of age or older, including pregnant mares, against Eastern, Western, and Venezuelan encephalomyelitis (EEE, WEE, VEE), West Nile virus (WNV), and tetanus, and against respiratory disease due to equine herpesvirus types 1 and 4 (EHV-1 & EHV-4) and equine influenza virus (EIV). The duration of immunity against EIV is at least 6 months and against WNV is at least 12 months. The duration for the remaining organisms has not been determined. For more information regarding efficacy and safety data go to productdata.aphis.usda.gov.
This product has also been shown to be effective against shedding due to EIV and against viremia, mortality, and neurologic clinical disease due to WNV.
Composition: Contains Kentucky lineage (KY/95), Florida sublineage clade 1 (OH/03) and Eurasian Newmarket/2/93 (NM 2/93) EIV strains. Amphotericin B and gentamicin added as preservatives.